Social Compliance
Social Compliance Assessment
Corporate Social Responsibility (CSR) and compliance to Social and Ethical code of conduct are considered as performance conduct of an organisation against Social standards related to human rights, labour regulation and working conditions. Acknowledging the complexity caused by legislative variations and industrial norms, we actively work with industrial partners and clients to enable organisations to implement social standards and meet compliance. Our services are available for various sectors, including textile, surgical, printing, food, minerals and aluminium. We cater for brand code of conduct, company’s own code of conduct and main compliance schemes including ISO26001, SMETA 4 pillar, SA8000, LSAS, RJC, ASI , OCS, RBA,PSCI , WRAP and BSCI.
We believe in enabling our clients all through their journey to deliver on social compliance be it their brand requirement, CSR initiative or Audit. Our services include;
- Gap analysis and improvement program
- Capacity building, training and coaching
- Systematic framework of continuous evaluation and improvement
- Performance measurement through assessments and audits

Beyond Social Audit (BSA)
The brands and retailers are engaged to improve social standards in their supply chains for many decades. The plethora of social audit schemes have increased burden on brands and suppliers without achieving desired social outcomes while the baseline issues still afloat. In terms of “lean manufacturing”, audits could be adding cost but not real value. This is a global challenge for all brands and industries. With the changing landscape of consumer demand, it was never more serious than today to evolve the industrial practices to a sustainable, reliable and impactful intervention.
We acknowledge audits can be effective tool of measuring the performance but nowhere near bringing the change in behavior and practices. To meet this challenge, we have developed a tool combining best elements of audit and education. This tool would serve as a generic platform for suppliers and brands to achieve change in practice and embed social and labour standards compliance. The tool is unique in its applicability and caters for all Social compliance schemes and code of conduct.
- Benefits for brands: Cost Effective, Change led, Sustainable, 1-2 days depending on factory size
- Benefits for suppliers: Happy workforce, free Education by experts, Increased productivity
- Benefits for worker: Fair Wages, Personal development, Workers rights,
The tool will enable companies to calculate return on investment (ROI). Research shows happy workers are 13% more productive and 28.4% less absent than unhappy workers. It will also assist companies to see exact benefits if 100% of their staff is happy.
It is a systematic approach to identify the gaps through self-assessment. The education plan is agreed with the factory before the visit in light with their potential non-conformities. This is followed by facilitating appraisal-based dialogue between workers representatives and management and agree on a plan of action. This will eventually built factory management and worker’s understanding on key issues and encourage them to take ownership for a sustainable solution.
This tool uses universal principles of social sustainability and will address CoC of any brand and any social audit scheme. This is an intervention-based model that will deliver desired outcomes instead of tick box exercise against a traditional checklist. This will address all concerns raised by the brands and help businesses flourish with happy and productive workforce.
UK responsible person will act on behalf of non-UK manufacturers to perform specific tasks regarding manufacturers obligations under these regulations. The key tasks and responsibilities of this role will include registering with the MHRA before a device is placed on the UK market. The responsible person also coordinates any requests between manufacturer and MHRA and immediately report any complaints to the manufacture. A full list of the responsibilities are set out in Part II (r. 7A), Part III (r. 21A) and Part IV (r. 33A) and in new Parts VIII and IX in regulation 77 (for medical devices) and regulation 146 (for in-vitro diagnostic devices (IVDs)) of the UK MDR 2002 (as amended by the UK MDR 2019).
Global Sustainability UK has been supporting business from many years by providing services as authorised Representative for UK and Europe including getting free sale certificates. Under new Medical Devices regulation in Europe, new responsibilities are added to the role of EU AR that includes being legally responsible to retain all technical documents and certificates regarding devices. AR should have access to any changes in the medical devices, maintenance records, and post market clinical follow up. It is also required that EU AR information should be robust and including in product communication materials and labels including Inserts, IFU’s and user manuals.
As your authorised Representative in UK and Europe, we will provide following services.
We will represent your business in UK and Europe, maintain your technical file as per MDR requirements, managing your electronic data submissions, liaise with competent authority on your behalf for any submissions or queries, getting you approved for CE mark by a competent authority, update all parties for any serious incidents, keep you updated for any changes in the regulations.

Sustainable Supply Chain (SSC)
Globally there are many cases where Brands with the best intentions, audit schemes and systems still face bad media reputation due to failing to perform on social sustainability standards, labour and human rights abuse in supply chain. Be it NIKE, Apple or Kick all unfolds the story behind the wall of child labour, forced labour and lack of provision of fair wages in global supply chains. Today transparency in supply chain is bigger challenge for businesses than Cost reductions, flexibility and speed of delivery.
GSUK offers a holistic solution that assures full adherence to labour standards and transparency in supply chain. Our program provides end to end capacity building and training across the supply chain. The selected programs will provide a paradigm to the team on maximizing their potential and enable them to add value to the organisation at various levels in Hierarchy.
It is 4 tier programs as described below:
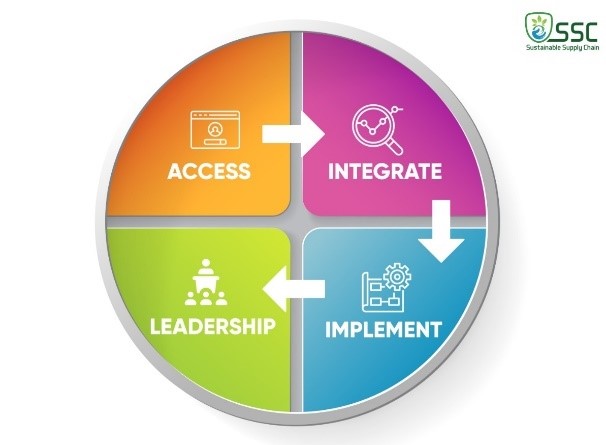
SSC looks at the process from raw material to the final consumption addressing Social, Environmental and Economical components of sustainability. This program is based on below Global Compact Principles

Our sustainable supply chain program will cover building on strengths, identifying gaps, providing training and comprehensive technical implementation of Sustainability standards and measures.

Modern Slavery Elimination Program (MSeP)
UN Sustainable Development Goals now call for an end to slavery by 2030. As a result modern slavery legislation introduced and impacted many countries including UK, EU, US and Australia. The legislation has created a challenge for businesses from Public and Private sector to address the issue.
We have devised a program to address all 7 elements of modern slavery in their supply chain. We support companies develop Modern Slavery statement, establish anti modern slavery framework and assurance system. Our model equip organisations with implementation tools to address modern slavery in supply chain.
Within this program we also support organizations for modern slavery audits, MSEP and other directives as required.
Our program assists organisations to deliver on robust modern slavery statement to comply with the legislative requirements, provide training, identify modern slavery risks and provide a Business continuity plan (BCP) incorporating Modern Slavery element. Our experts have worked closely with government agencies like GLA to devise a structure appropriate to organisation needs and deliver actionable program on Modern slavery.
